June 2, 2007
preface
Verification center for organizations to carry out the drug registration production on-site inspection, inspection of drug GMP certification, GMP inspection, flight check, imported drugs outside production site inspection, circulation of check and observation check a total of 434 items.
Section 1 the on-site inspection of the pharmaceutical registration
First, check the base case
In 2016 received 29 inspection tasks (including multi-stage check), 21 from administration of drug approval center (hereinafter referred to as the "human medicinal center"), eight for inspection tasks. A total of 178 people from the 43 inspection teams were conducted on-site inspections. There were 42 completed on-site inspection reports, including 34 of them, 81 per cent. The number of applicants who fail to sign up for registration is 19%.
Second, discover the main problem
In 2016, on-site inspection, the individual enterprise report data cannot be traced, the data is not real data reliability problems still outstanding, also found inadequate process validation, production process is not stable, the production process or approved by the parameters and inconsistent.
The details are as follows:
(1) data reliability issues
The test data can't be traced back to the source. There is no such thing as keeping the sample and the stability test. The samples of the samples of the samples of the specimens are not inspected, and the samples of the samples of the specimens are not inspected and the records are not provided.
The batch production records are not true and incomplete, not in conformity with the declaration data.
The process verification is not sufficient and the production process is unstable
Varieties of process validation is not fully, the enterprise in the dynamic production, serious deviation, batch production yield and validation batches deviation is larger, the production equipment control system is not stable, production lines, part of the equipment is not fully satisfied the production requirement.
(3) the production process or the key process parameters, the contents of the inner and the materials are inconsistent with the contents of the verification, and the evaluation is not carried out
The production process is inconsistent with the production process of verification/declaration.
The key process parameters are not consistent with the approved/declared production process.
The manufacturer of the inner and packing materials is inconsistent with the registration declaration, and the enterprise has not conducted the comparative study.
(4) not carrying out the necessary deviation investigation
In the case of major anomalies in dynamic production, the investigation of individual enterprises is inadequate, and the root cause is not found. The three batches and the dynamic production batch results showed that the yield difference between the four batches was large, and the reason for the analysis was not analyzed.
Section 2 GMP certification examination
First, check the base case
According to the state food and drug supervision and administration bureau about the failed drug production quality management standard (revised in 2010) certification enterprises to stop production and lowering of sterile drug certification announcement no. 285 (2015) "the spirit, since January 1, 2016, the state food and drug supervision and management of administration no longer accepts the drug GMP certification application. Continue to organize the on-site inspection and approval certificate for the certification application that has been accepted.
In view of the above, a total arrange inspection 16, 2016, 16 received on-site inspection report, complete audit 14, 12 of pharmaceutical production enterprises through the drug GMP certification examination, two pharmaceutical producing enterprises failed drug GMP certification examination, issued a warning letter 5 enterprises; Two other companies have completed their GMP certification, but the certification process has been suspended because they have not yet been registered.
Apply for certification of dosage forms including large capacity injection three times, small capacity injection, producing injection three times, three times 1 1 powder injection, radioactive drugs, vaccine products two times, and other types of biological products three times.
Second, discover the main problem
Found 220 defects, including 23 major defects and 197 defects. Quality control and quality assurance of these 41, 32 file management defects, defect 24 organizations and personnel, equipment defect 23, defect 21 items of qualification and validation. The survey found that the distribution of defects was identical in 2015.
The two external diagnostic reagents produced by this year's certification inspection are not approved, and the main questions are as follows:
Quality management system. The quality management system cannot be effectively run and the production and quality requirements of the product are not guaranteed. The personnel is very mobile, the professional staff are short of, the training is not in place, can not meet the daily production quality management requirement; The documentation is not operational and the data records are incomplete; The relevant changes are not controlled according to the change procedure.
Verification and verification of work. Failure to perform process verification on all products approved for GMP and not in place for clean verification of public facilities; Partial verification records are incomplete; Partial revalidation work is not carried out in accordance with the requirements.
Section 3 drug GMP follow-up check
First, check the base case
The 2016 inspection center announced a follow-up to 215, 228 of them. Among them, 58 times, the remaining 170 were inspected. In addition, there were 21 follow-up tests for the provincial certification of sterile pharmaceutical production enterprises, and 13 double-check inspection. A total of 204 inspections were completed throughout the year.
Of the 12 companies that tracked down the number of companies that were not approved, they accounted for 6.1 per cent, with 59 out of 59, accounting for 29.6 per cent.
In check by 12 companies, 2015 sample unqualified enterprises have five, double random check enterprise 4, 2 citicoline sodium injection production enterprises, 1 bone peptide injection production enterprises.
(1) the variety of quality announcements of the year 2015 shall be examined
A total of 10 companies were tracked, five of which were not approved, 50 per cent, and four companies issued warning letters.
Double check
For the implementation of the state council, make innovation, regulatory requirements, in accordance with the unified administration of the deployment, drug double random inspection system to run for the first time in December 2016, adopt the method of layered double random select 13 companies to carry out the track inspection, including 2 3 chemicals, active pharmaceutical ingredients, eight Chinese medicine, located in nine provinces. A total of four companies were not approved, with a passing rate of only 69 per cent and a warning letter to three companies.
(3) vaccine manufacturers
For pharmaceutical GMP certificate of 38 vaccine production enterprises included in the trace examination in 2016, in addition to 1 for pharmaceutical production license and pharmaceutical GMP certificate back in 2014, 1 in 2015 to address drug GMP certificate change production application to not perform inspection, conducted among 36 vaccine production enterprises to track inspection. The results were all passed, with seven companies warning letters. One of the enterprise because of the change analysis detector, preliminary thought is a serious defect inspection group site, expert discussion that late changes before and after the detector method principle is consistent, quality risk is low, require enterprises to carry out further methodology verification, as the main defects. Overall, the vaccine production quality risk is controlled, the production and quality management of production enterprises are more standardized.
(4) production enterprises of blood products
26 blood products production enterprises included in the trace examination in 2016, a total of 25 blood products enterprises to carry out the track inspection, otherwise 1 companies for production improvement did not check. The results were all passed, and the four companies issued a warning letter. In general, the production quality of blood products in our country can be controlled, the production and quality management of production enterprises are more standardized, and the supervision of individual enterprises should be strengthened.
(5) enterprises that sent warning letters in 2015
A total of 32 companies that sent a warning letter in 2015 were followed by a follow-up check, although the company was largely compliant, but it still sent a warning letter to 14 companies.
(6) after the authentication, it shall be examined by the pharmaceutical producing enterprises which are certified by the provincial level
A total of 21 provincial-level pharmaceutical producing enterprises, all approved by the provincial government, issued a warning letter to six of them. Through the inspection, the scale of the inspection of the provincial bureau is strictly controlled, and the functions of the certification inspection are carried out smoothly.
(7) special examination of high-risk varieties
This year's focus on bone peptide, fructose ErLinSuanNa, citicoline sodium injection of three products such as the track inspection, high-risk variety special inspection plans for 114 times, there are 47 as companies failed drug GMP certification (revised in 2010), variety, long-term production approval number transfer reason not checked, the actual check 67 times. An injection of 1 osteopeptide injection company and 2 cell phosphocholine sodium injection products were not approved by the company and sent a warning letter to 21 companies.
Second, the main problem found
(1) the overall situation
In article 204 check-up found 204 defects, 22, and serious defects of the main defects of 212 items, 2026 general defects, compared with the GMP certification in 2015, the track inspection serious defects number increased.
In high-risk species varieties of long-term production in the enterprises of the special inspection or not through drug GMP certification (revised in 2010) the phenomenon is more prominent, inspection found that some of the common problems are as follows:
There is a discrepancy between the manufacturing process and the registration process.
2. The data reliability problems still exist, including forging production records, inspection records, modify the data without authorization, to paraphrase in production, equipment, material records related content correctly.
The process validation is not sufficient, especially when the production batch has not been carried out.
4. The normative data management problems, mainly reflected in the system permissions, audit tracking function, data files and modify and delete permissions to control, and to delete the data and choose to use the data of no control and reasonable explanation.
The implementation of the computer system, the confirmation and verification of the two appendices has a certain gap in the requirements of the regulations, and the problem is more.
6. The management of deviation and change is relatively weak, mainly reflected in the deviation of the cannot effectively identify and record, lack the necessary evaluation and verification of the change.
(2) enterprises that are not eligible for inspection in 2015
There were 11 major defects found in 10 enterprises, the main defect was 27, and the general defect was 84.
The main problems found are: the production process is inconsistent with the registration process, the data reliability problem, the process verification problem.
(3) double check
Found serious defect 5, major defect 24, general defect 123.
Found that the main problems include: falsified records, products quality and safety hidden trouble, data reliability problems, problems of process validation, the material management is not standard, the risk of pollution, confusion and error cleaning not thoroughly, can not effectively prevent contamination and cross-contamination.
(4) vaccine manufacturers
Found the main defect 38, general defect 383.
The main problems found include:
1 device aspect. The injection water preparation system enters the stainless steel line of the injection water tank and the valve is too long between the water machine.
Materials and products. The record for the destruction of finished products shall be refined; Missing individual cargo CARDS; No background information on the whole genetic sequence of the mycotoxin species that was produced.
File management. Individual file regulations are not specific and operational, and the contents of the document are slightly different from the actual documents. Individual records are incomplete; The design of batch production records is unreasonable, and the actual operation is not timely.
Quality control and quality assurance
(1) quality laboratory management: failing to ask for the necessary inspection data and graph from the organization that received the entrusted inspection.
(2) deviation processing: the training and execution of the relevant documents of the enterprise deviation is not in place; The individual deviation failed to initiate the investigation in time; A small number of deviation factors are analyzed and corrective measures are not put in place, and the potential impact on the quality of the product is not fully evaluated.
(3) change control: the change is not processed by the change process and the application for registration is applied. There is no assessment or assessment of certain changes.
(4) supplier management: the audit content of key material suppliers needs to be refined, and the content of the supplier's audit content needs to be improved.
(5) product quality review: annual quality review should be carried out according to the variety, and a lack of effectiveness analysis of CAPA in retrospect; The analysis of annual product quality review analysis can be further refined.
Computer systems. Enterprise management developed a computerized system of file system, but has not fully carried out in accordance with the system of classification management, the existing conditions do not conform to the file, not take effective measures to reduce risk; The quality inspection room HPLC is required to set up the requirements for the login interface.
(5) the production enterprise of blood products
A total of 25 enterprises were examined, and the defect was found in 0, the main defect was 13, and the general defect was 241.
The main problems found:
There is a lack of training for some job operators.
There is no effective control of pollution and cross-contamination in the plant and facilities.
Equipment. Some equipment calibration fails to cover the actual use; Some of the inspection instruments are not properly calibrated or calibrated, and the records are incomplete. Partial equipment identification is not complete.
Materials and products. The information in the cold precipitation label is not sufficient and the label is not fixed.
Validation and validation. The culture medium simulated the filling experiment did not specify the specific requirement such as the requirement of the intervention and the standard.
File management. The contents of the document such as process rules and procedures are not specific and the regulations are not specified. The records are not filled in time, incomplete or incomplete.
Production management. A solution of sodium caprylic acid, which was added before the inactivation, and a lack of control measures for microorganisms; Do not confirm the setting time of the sedimentation dish at the a-level laminar flow.
Quality control and quality assurance
(1) quality control laboratory management: the contents of the inspection and receiving and receiving of the Taiwan account are incomplete, and the receiving information of the sample is not recorded in the intermediate and semi-finished products; Unpurchased petri dishes for environmental monitoring for testing; The aseptic experiment did not set the negative control against the pharmacopoeia;
(2) product stability inspection: intermediate products have a validity period, but there is no continuous stability investigation or verification data support;
Change control: change control management is not in place. Incomplete assessment or assessment of partial changes;
(4) deviation processing: individual deviation fails to initiate the investigation in time; Partial deviation investigation and corrective and preventive measures are inadequate;
(5) supplier management: the supplier fails to be included in the list of qualified suppliers for cold precipitation.
(6) enterprises that sent warning letters in 2015
There were 32 major defects, 32 major defects and 328 general defects.
Found that the main problems include: data reliability problems, not to record and investigation of deviation, aseptic security shortcomings, relevant qualification and validation work is inadequate.
(7) sterile pharmaceutical production enterprises with provincial certification
Examine 21 enterprises to find serious defect 0, major defect 15, general defect 209.
The main problem of discovery was that the production risk assessment was inadequate for the small capacity injection. Clean verification of single varieties and not all varieties of the enterprise; Partial inspection records are incomplete; The verification and auditing of computerized systems have not been carried out; Improper material management, confusion, risk of error, etc.
(8) special examination of high-risk varieties
A total of 61 enterprises were examined to identify serious defects, 64 major defects and 658 general defects.
The main problems found are as follows:
1. The injection of bone peptide
(1) purchase from the meat products company, not purchase and acceptance according to the regulations. After the material arrives, the inspection report issued by the supplier shall not be taken by the SOP and checked by the supplier. At the time of inspection, the temperature monitoring record for the four parts of the animal's dismembered parts is not taken.
(2) the virus inactivation of the compound bone peptide was untested; No data support was given for the time stored in the four limbs of the pig and the time of the compound osteopepine injection from irrigation to sterilization.
(3) the continuing stability test scheme for regulated activity index and related safety index and inspection, such as: allergic experiment, pyrogen, abnormal toxicity and so on.
Sodium fructose sodium phosphate injection
The standard of microbiological standards in the internal control standards of the sodium fructose sodium phosphate group has not been amended in accordance with the relevant regulations of the Chinese pharmacopoeia in 2015. The content of the compound enzyme used in the determination of the content of sodium fructose sodium phosphate is still used in the determination of the content of the raw materials after the expiration date.
Sodium cytophosphate sodium injection
The standard of the internal control of sodium phosphite sodium in one enterprise is unreasonable, and the project of adding toluene residue to the quality standard of the original pharmaceutical production enterprise is not reasonable. The sodium chloride injection of a certain enterprise has not been tested separately for the raw materials of different manufacturers. The compatibility test data of the test of compatibility test were not provided.
Section 4 drug flight examination
First, basic situation
In 2016, the general administration of the general administration of the general administration of the general administration of the general administration of the general administration of the general administration of China (sepa) has conducted 45 inspections and completed and reported the results of the drug GMP flight 39 times, other than in 2017. Has reported the results of 39 times, including Beijing, jiangsu, guangdong and other 20 provinces (municipalities), including 9 biochemical drugs production enterprise, 20 Chinese medicine production enterprises, nine general chemical production enterprises and 1 blood products manufacturing enterprises.
The task of the 2016 flight inspection mission, which comes from the general administration of administration of medicine, 33, is a total of 6 tasks from the bureau of drug registration. The general administration's administration of drug control, which accounts for about 85 percent of the mission, is the main source of flight inspection. Chinese medicine accounted for 51 percent of all flight tests and 23 percent of general chemical and biochemical drugs. A total of 21 drug production tests were not approved in 2016, accounting for 54% of the total. Fourteen of them were advised to take the drug GMP certificate, and ten were advised to file a case investigation, and seven companies were ordered to recall their products. In GMP flight tests, there are more problems with traditional Chinese medicine and biochemical drugs. The general administration has already handled the problems found in flight inspections in accordance with the law.
Second, the main problem found
(1) the main problems discovered by Chinese traditional Chinese medicine enterprises
According to traditional Chinese medicine production enterprises, in 2016 sent 66 person-times of 18 inspection teams to 20 companies in the flight check, involving letter-inquiring-and-accusing 7 companies, artificial bezoar producing three enterprises, products containing bezoar class 7 companies, exploratory study inspection found that the problem of 3 companies, Chinese medicine yinpian 1 companies.
Class 20 Chinese medicine manufacturing enterprise of the flight check, including 12 companies do not conform to the requirements of the drug GMP, for 3 companies over the issues concerning the ShengJu processing, to the companies issued a warning letter, 1 companies related to production, conform to the requirements of the three companies.
Chinese drug manufacturers
(1) changing the process problem without authorization is more prominent.
In order to reduce the cost of production, the phenomenon of pre-processing and extraction of the drug is an outstanding issue. This year for exploratory survey found that the problem to carry out the flight check, all is the enterprise will be part should be extracted in the prescription of Chinese herbal medicine is not according to the technical process to extract, but after feeding directly.
(2) the management of traditional Chinese medicine and Chinese medicine is chaotic.
Individual proprietary Chinese medicine production enterprises, in response to the pharmaceutical supervisory and administrative departments at all levels of the supervision and inspection, make warehouse material parameter and in-out warehouse records, according to the traditional Chinese medicinal materials production retracing the usage, dosage and on-demand fabricated material parameter.
(3) the purchase of Chinese medicinal herbs, Chinese medicine and Chinese medicine is not strictly examined, and the data reliability is problematic.
Traditional Chinese patent medicine production enterprise product variety, involving much more varieties of traditional Chinese medicine, Chinese medicine yinpian, due to the configuration of precision analysis instruments and QC personnel were not adapted to the scale of production, all can't promise to buy Chinese herbal medicine and Chinese medicine yinpian swarms, causes on the part of the batch of traditional Chinese medicine, Chinese medicine yinpian without a full inspection, or use a graph multi-purpose behavior to check.
Artificial cow-yellow
Some companies can't organize production in accordance with the pharmaceutical GMP requirements, especially for artificial bezoar upstream industry chain to a serious shortage of supplier audit and management requirements, supplier processing workplace health conditions, raw material source cannot be traced, machining process is not controlled. The main problems include: (1) weak supplier management; (2) the validity of the results of microbiological examination; (3) not included in the quality assurance system.
Chinese medicine drink
The contents of the products are highlighted, the problems of dyeing and weight gain have occurred, and the production records are of dubious validity. The main problems include: (1) the production record is not true; (2) to be involved in the distribution and sale of foreign food and drink tablets; (3) data reliability issues.
(2) biochemical drugs
"Monosialic acid tetrasin sodium glycosidine sodium"
2016 check four "single sialic acid has four sugar ganglion glucoside ester sodium" production enterprise, found in the raw material quality and supplier management has certain risk. The main questions include:
(1) supplier management needs to be improved, and the management of the suppliers to the suppliers cannot guarantee the effective traceability.
(2) insufficient data management for the monitoring of raw material cold chain transportation.
"Injected with the growth hormone of liver cells"
2016 check four "injection in promoting liver cell auxin production enterprises, the two companies are back" medicine GMP certificate ", to the other two companies sent a warning letter. The main questions include: (1) making up a record file; (2) data reliability issues; (3) inconsistent with the registered manufacturing process; (4) the quality of liver raw materials cannot be effectively guaranteed.
Section 5 overseas examination of imported drugs
First, check the base case
(1) an overview of the annual inspection mission
According to the general administration's "letter to the implementation of the on-site inspection plan for the import of imported medicines in 2016", etc. There are 49 overseas missions in 2016.
The 2016 inspection mission involves 19 countries. Number of varieties of Europe, North America and other regions, drug quality high risk in regional check varieties of India, Vietnam and other countries also occupy a certain proportion, and increase inspections for South America and Australia regions.
2. Follow the principle of inspection service to review the examination and approval, both listed product safety requirements, check the percentage increase in variety, to declare in registration and more clinical, declare the production, application stage products are included in the scope of inspection, check the cause of the listed products mainly for the port inspection problem, increase the risk of adverse drug reactions monitoring high varieties.
The inspection task shows the characteristics of comprehensive and dosage forms, and increases the strength of the inspection of chemical agents. There are 40 chemical medicines, including injection, solid preparation, implantation and nasal spray, and 3 of them, and 6 of them. 11 vaccines, blood products, and biological products; Four plant medicine.
(2) check for implementation in 2016
Because years administration working arrangement, readjust the plan time overseas examination in 2016, production enterprise production scheduling changes lead to a total of 2016 completed 15 varieties of inspection tasks, including on-site inspection of 7 varieties, not through three variety, accounting for 42% of the check number; During the inspection of the organization, the enterprise voluntarily revokes the import registration certificate of the breed, or has been retried, accounting for 17 percent of the total annual plan. Another 21 species due to the enterprise production scheduling, in the first quarter of 2017 to complete inspection, the remaining 12 varieties, as companies can't accept the inspection in the first quarter of 2017, has been incorporated into the plan for overseas examination in 2017.
Second, the main problems discovered by overseas inspection
Of the seven species examined, three species did not pass, and the rate of passing was higher than in previous years. The defects were found in 117 defects, including 3 major defects and 18 main defects. The problem focuses on quality control and quality assurance, material system, change management, etc. The major defects are the consistency of production process and the problem of data reliability. All problems discovered abroad are handled according to law.
The main and serious questions are as follows:
The actual manufacturing process and the production site are not in conformity with the registered declaration, the major changes are not reported to our country but have been executed.
There are major problems in data reliability, which seriously affect the quality of the products.
The number of non-on-site inspections is increasing.
Section 6 inspection of drug circulation
First, check the base case
2016, total bureau of national food and drug supervision and administration of illegal drugs circulation management behavior to carry out the centralized control, through the enterprise comprehensive rectification, ShengJu supervision, general administration of implementation of flight test methods such as further improve and standardize the drug circulation order, crack down on illegal conduct. In the whole year, there were three batches of the pharmaceutical wholesale enterprises. The results of the flight inspection shall be announced by the general administration.
Inspection group on the basis of the general administration of medicine circulation field about regulation of illegal conduct announcement no. 94 (2016) and "the drugs management quality management standard" flight check for relevant enterprises. It is found that the illegal and illegal behaviors of drug circulation enterprises are more common.
Second, discover the main problem
(1) the enterprise has been in violation of the state administration's announcement of the state administration of general administration 94.
There are major problems:
Not monitoring and storing, transporting and conducting temperature and humidity according to regulations.
2. When purchasing and selling drugs, certificate (license), ticket (notes) invoice, along with the cargo counterparts, zhang (zhang, physical CaiWuZhang), physical (drugs), money (payment) can't be corresponding consistent with each other; The drugs are not in the library, the books are set up, the medicines are not included in the enterprise quality system management, the use of the bank personal account for the business transactions and so on.
3. The source of counterfeit drug purchase, fiction drug sales flow, tamper with the computer system, temperature and humidity monitoring system data, to hide the real drug distribution records, bills, documents, data, etc., drug purchasing and selling stock record is incomplete, not real, management behavior can't back.
(2) the enterprise is in violation of the general administration of quality management of drug administration (GSP).
The defects of GSP are mainly concentrated in general, storage and maintenance, sales and so on. Major problems:
There is a false and deceitful act in the absence of the law.
To change the business registration address without authorization; Storing and storing drugs in a warehouse under the operating license; To provide the conditions for the illegal marketing of drugs for others; The origin and the direction of sales of fictitious medicines; Hiding bills and providing false information; Self-report is not true; Falsification of tax returns; Tampering with temperature and humidity monitoring data, etc.
Do not store the medicine according to the regulations, do not monitor and control the temperature and humidity of the warehouse.
In order to buy and sell drugs, the ticket, the account, the goods and the funds may not be identical, and the sale of special drugs shall not be enforced by the state.
Section 7 GMP inspection of foreign institutions
First, check the base case
Verification center, a total of 2016 organizations for pharmaceutical production enterprise of observation check 81 times, 76 involved enterprises, covering zhejiang, shandong and other 20 provinces (municipalities), zhejiang, shandong, jiangsu, guangdong, hubei, hainan, hebei accounted for 80%, compared with the previous year, the proportion between the provinces changes slightly.
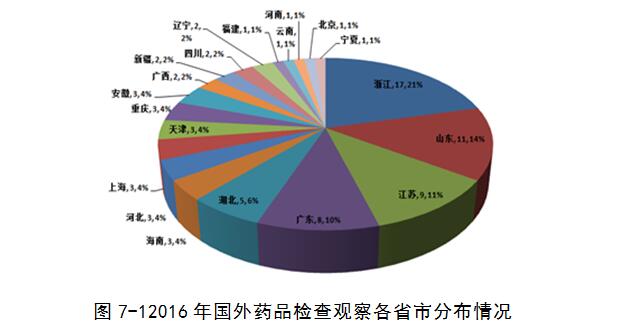
2016 observation check including inspection agencies including the world health organization (WHO), the European medicines quality council issued (EDQM), the United States food and drug administration (US) to the FDA, Hamburg, Germany health and consumer protection department (BGV), the Brazilian health supervision bureau (ANVISA), the French national medical health and safety administration (ANSM), and other 12 international organizations or foreign drug regulatory department. Nine pharmaceutical companies were found to be seriously flawed, and they did not pass the on-site inspections (accounting for about 11 per cent of the total).
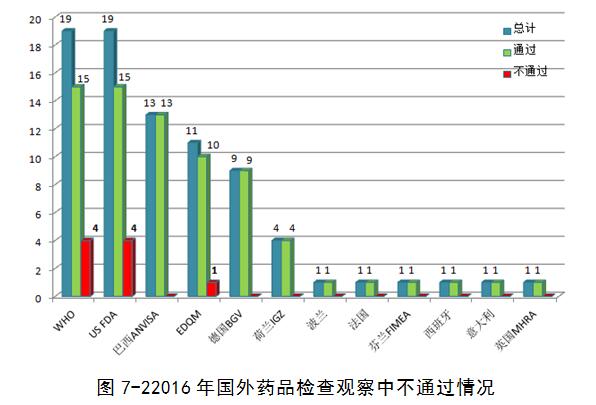
Compared with 2015, the ratio has risen. In nine not by examining the enterprise, the most serious defects of data reliability issues (including repeat test to, change system time after testing qualified, delete data, delete the audit tracking, selective use of data, modify the name of the electronic data, test sample and record is not timely, not real, loss of data and records, file record control is insufficient, etc.), some enterprises involves material standards unreasonable, inadequate measures to avoid cross contamination, etc. Overall, the data reliability problem is more outstanding, this also is in 2016 domestic enterprises to accept foreign check is not the main reason for the pass rate rise, also reflects the change tendency of the current drug inspection.
This annual review examined 172 drugs, including 119 raw materials, 23 oral solids, 19 injections and five biological products. In 81 involved in the inspection of API 62, accounts for about 69% of all inspections, involving the oral solid preparation check 12 times, about 13% of all inspections. The proportion of raw materials was the largest, followed by oral solids, and relatively few other types of dosage forms. Compared with 2015, the proportion of other forms of dosage (including sterile preparations, biological products, etc.) has increased from 10 per cent to 18 per cent in 2015, up from 10 per cent in 2015.
SSecond, the main problem found
Observation record check the work of the communist party of China in 2016 found that the defects of item 1108, according to the 2010 edition of GMP text chapters classifying defect item analysis found that: the quality control and quality assurance, file management, equipment, materials and products, qualification and validation, plant, and the six categories of defect accounted for 88% of the total defects. Compared with 2015, the file management part defects by the fourth rise to second place, in the current enterprise gradually to strengthen the management of data reliability environment still grew by about 7% (from 10.5% to 17.3%), which fully reflects the current foreign inspection focus on data reliability degree and strict requirements.
In the foreign drug GMP inspection, the "quality control and quality assurance" portion of the defects accounted for 27.3% of the total defect number, ranking first. Main problems focus on the management of the laboratory computerized analysis instrument, deviation handling and CAPA, product quality review analysis, change control, OOS/OOT result processing, lab did not follow the rules of the control program, microbiological examination management and quality risk management, stability test and so on. "Document management" comes second part defects, defects have focused on record integrity and traceability, lifecycle management, file integrity, record operation four aspects. "Equipment" part of the defects in the third place, which the use of equipment and clean, calibration, maintenance and repair, the defects of four aspects of the water system management part of the 83.6% of the total defective item. "Materials and products" section of the defect item on supplier management, material and product identification, material flow management, material and product standards compliance, and release management. The "validation and validation" defects mainly include validation of scientific, validation, validation, and recording. The defects of "workshop and facilities" part mainly includes the environment control, warehouse management, measures to reduce contamination and cross-contamination, plant facilities management and so on four aspects of life cycle.
Three, different orgnaization drug GMP examination analysis
Check the content, although the focus of the different drugs GMP inspection agencies inspection has certain differences, but through the observation of 2016 check the defects analysis found that the quality control and quality assurance, file management, equipment, materials and products, qualification and validation, plant, and facilities and so on six parts of more defects. In the check article put forward the defects in the final report number, issued by the EDQM, WHO check defect data is relatively more, the average check article about 20 defects, defects in the project of problems found during the inspection process are described, after finishing writing the final inspection report (usually a month or so). Article put forward the defects in the US FDA in the check number is relatively small, the average check about 7 defects, not all of your problems will be found in the process of checking all defects as the final item, inspectors according to the problems found in combination with the final defects after judgment on the risk of product items, and will be held in check by the end of the time (483) to inform companies in writing.
|
