Medical network May 26 - yesterday, Merck (MSD) announced that the FDA has accepted review its complement biologics license application, use of therapy pie, PD - 1 single resistance (Pembrolizumab, Keytruda) treatment has accept two or more chemotherapy for recurrent or advanced gastric cancer or stomach esophagus adenocarcinoma patients. It is a major development in the field of gastric cancer treatment, like the apatinib tablet, which was developed by jiangsu hengrui in its early years. What is the implication of this market for the total of 28 billion yuan of gastric cancer in China?
Held in Beijing in April 2017, according to the 12th international congress on gastric cancer disclosure, China's new cases per year is about 680000 cases of gastric cancer, accounting for about half of the total global disease. Most of China's stomach cancer patients are in the middle and late stage of diagnosis. It's a very clinical and very difficult malignancy.
It is reported that people over 40 years of age have high levels of stomach cancer. Unhealthy eating habits are an important risk factor for stomach cancer. Improving health management and promoting healthy lifestyles and promoting the listing of new drugs is one of the major initiatives in the long-term plan for chronic chronic disease in China.
Anti-tumor treatment entered the new age
The global anti-cancer treatment has entered the era of precision medicine, which has promoted the rapid development of the market. According to the U.S. version of the precision medicine program, the key to the field of anti-tumor medicine is gene sequencing, cancer, and personalization. Chemotherapy drugs have become an important category in clinical practice.
In the past two years, the U.S. FDA has approved few drugs to treat stomach cancer, and the medical community is feeling helpless.
And just yesterday (May 24), Merck announced that its resistance to therapy pie, PD - 1 single resistance (Pembrolizumab Keytruda) treatment has to accept two or more chemotherapy regimens recurrence of advanced gastric cancer or gastric adenocarcinoma of the esophagus connected supplement biologics license application priority review by the FDA.
This is another bioengineered drug after the FDA approved the company's antibody drug, Ramucirumab, in April 2014. Remo reed sheet resistance is one for the specificity of blocking vascular endothelial growth factor receptor 2 (VEGFR2) and angiogenesis related pathways downstream of humanized monoclonal antibody, used in the treatment of advanced cancer of the stomach or gastroesophageal junction adenocarcinoma. But the FDA has not approved a new drug for treating stomach cancer for nearly five years.
Thankfully, jiangsu hengrui medicine independently developed the national class new medicine 1.1 mesylate path for piece, in November 3, 2014 issued by the state food drug safety administration (CFDA) register new drug certificate and production. Path for Nepal is the world's first in advanced gastric cancer proved safe and effective targeted small molecule anti-angiogenesis drugs, is also a standard of advanced gastric cancer after failure of chemotherapy, significantly prolong the lifetime of single medicine. This is another major breakthrough in antitumor fields in our country, also for advanced gastric cancer targeted drugs market added a new vitality, in patients with gastric cancer ushered in the dawn of the east.
The chemotherapy market for gastric cancer is not lonely
According to the world health organization's global statistics report, the annual incidence of gastric cancer in the world is 13.86 per 100,000, second only to lung cancer. Our country is the high hair area of gastric cancer. The latest statistics show that there are about 680,000 new cases of stomach cancer in China each year, up from 44.65 million in 2012, with an average annual growth rate of more than 13 percent. The death rate of stomach cancer in China is four to eight times that of developed countries in the United States and Europe. It is a major disease that is currently endangering the health of our people.
Stomach cancer is a cancer that begins in the stomach tissue and develops slowly for years. So far, the cause of cancer of the stomach is not clarified, helicobacter pylori infection, nitroso compound and high nitrite and dicarbonyl compounds, as well as fungi and genetic gene is one of the important factors affect gastric cancer occurs. The incidence of gastric cancer is high with the development of the global aging society and the influence of human diet and ecological environment. Abroad, the number of new cancer cases in the world is expected to rise to more than 19 million by 2025. And the cancer situation in Asia is not trivial, and it will be the focus of growth. Higher incidence of gastric cancer in China's coastal urban areas and the northwest. Therefore, it is important for the global medical community to pay enough attention to gastric cancer.
With the introduction of new drugs and the advance of combination chemotherapy, cancer mortality in China has been decreasing in recent years. The number of men fell by 21.4% a year and women by 21.1% a year. But China's huge population and ageing population are still driving the market for anti-cancer drugs. Experts predict that by 2030, the number of new cancer cases in China will exceed 5 million, with an annual death toll of 3.86 million. The area of malignancy is still a high-profile market.
The constant battle between demons and the tao
In recent years, there has been a change in the market for gastric cancer chemotherapy after the publication of the guidelines on the standardization of gastric cancer. In the traditional with platinum, on the basis of fluorouracil and its analogues chemoradiation, puts forward a new adjuvant chemotherapy, postoperative adjuvant chemotherapy and palliative chemotherapy for a variety of solutions.
According to m Intranet China city public hospital chemical medicine terminal monitoring and analysis system (HDM), according to data from 2016 key cities for the domestic public hospital anti-tumor and immune modulators medicine market has reached 23.9 billion yuan, a 10.30% year-on-year growth, including gastric cancer chemotherapy drug market is 6.556 billion yuan, year-on-year growth rate of 12.24%, occupies the key city public hospital anti-tumor and immune modulators drug use 27.43% of the market, many more than 20 drugs for the treatment of gastric cancer. It is estimated that the overall market for chemotherapy for gastric cancer in China has reached 28 billion yuan.
At present, the main varieties used in the treatment of gastric cancer by dorsey yew series, fluorouracil derivative, platinum, antitumor antibiotic, camptothecin derivatives, and leucovorin drugs; New entrants to the field of antibody drugs and small molecular-targeted drugs are extremely attractive.
Antitumor targeted small molecule drugs and biotech drugs has become the mainstream of global cancer market, but is still a little on the treatment of gastric cancer, currently only targeted drugs are marketed by roche bead sheet resistance, lilly remo reed sheet resistance (Cyramza), China hengrui path for.
The market for gastric cancer chemotherapy is a bit green
It is well known that the early symptoms of gastric cancer are atypical and stealthiness, and most of the stomach cancers come from the inner lining of the stomach (mucous membrane). And in China, the routine examination of the gastroscopy is far from being widespread and important enough.
Figures show that about 60% ~ 80% of the domestic when the patient has been in the middle-late stage, after the operation effect is very ideal, and the prognosis is poor, in the absence of effective drug therapy, combined treatment of middle-late gastric cancer still show the helpless. The elderly are the main body of the stomach cancer, and the overall mean survival is less than one year, and the five-year survival rate is less than 20%.
In human and malignancy, stomach cancer is a clinically difficult disease. Over the years, the principle of comprehensive treatment has been divided into surgical treatment, radiotherapy and chemotherapy and traditional Chinese medicine. Especially for preoperative or postoperative gastric cancer were recommended based synchronization with treatment, but drug therapy and postoperative chemotherapy drugs are actually showed a dismal.
For years, the clinical treatment of stomach cancer was an astringent market. Humanity in the 21st century has made certain progress, in the traditional fluorouracil and cisplatin, table doxorubicin took a big step, on the basis of the formation of the current clinical commonly used oral fluorouracil carbamate drug capecitabine and cisplatin new derivative and yew series oxaliplatin into drugs and therapy of alternate and difference. Carpei-cisplatin is an important part of the treatment market after the complete removal of gastric cancer. With the advent of targeted drugs, new "cocktail" therapies will have a similar effect.
The gastric cancer treatment drug tiggio is growing rapidly
In the anti-gastric cancer drug treatment market, the anti-metabolism-drug fluorouracil line is the base drug in chemotherapy. Key cities for the domestic public hospitals use main varieties for gonow capsules and tablets, capecitabine tablets, preparations, for adding fluorine fluorouracil, deoxidation floxuridine capsule, compound fluorouracil oral liquid, uracil for fluoride tablet, comer fluoride tablet, etc.
Fluorouracil is a synthesis of the first generation in the 1960 s metabolism of drug resistance, is currently widely used anticancer drugs, promotes the generation of drug fluorouracil series products into the market, is the groundwork for gastric cancer chemotherapy drugs.
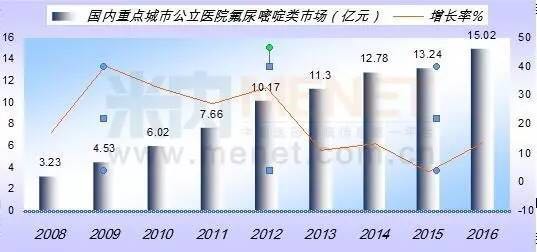
According to the HDM system, the market for the use of fluorouracil in public hospitals in China's major cities in 2016 has exceeded 15.02 billion yuan, up 13.44 percent year on year. The top priority of the fluorouracil line is giogio, which is the leader in this category with a 50% share.
Tigio is an oral agent for fluorouracil and is made up of potassium fluoride, gimedimidine and otialate. Guangdong dapeng LNG company LTD in Japan in 1999 approved the Japanese developed for gonow treatment of advanced gastric cancer, then extend for head and neck cancer, colorectal cancer and non-small cell lung cancer chemotherapy. Japan's dapeng capsule has been registered in China with the name "aisivan". Currently, most standard protocols for treating gastrointestinal tumors contain fluorouracil and its derivatives. Clinical data show that for gonow in late digestive system malignant tumor chemotherapy, there are more than 80% of the cases used for gonow, treatment effectiveness 44.6%, is a safe and effective anti entity carcinoma of oral drugs.
In 2014, the CFDA approved the registration of geo tablet for fuzhou haiwang fuyao pharmaceutical. At present, the domestic tiggio has been monopolized by shandong new era pharmaceutical, Zulu pharma, jiangsu hengrui medicine and fuzhou haiwang fuyao pharmaceutical.
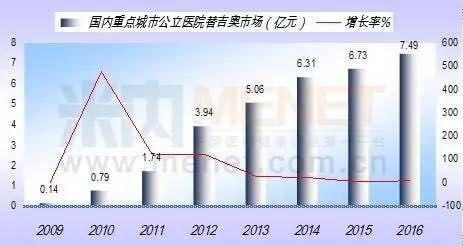
According to data from the HDM system, the amount of drug used for gigio was 749 million yuan in 2016, an increase of 11.27% year on year. The capsule was 99.96% and the tablet was 0.04%. The overall market for giggio, the domestic grade hospital, has reached 26.65 billion yuan.
84.42% share of domestic for gonow to dominate the market, of which shandong new time pharmaceutical for gonow capsule weikang to account for 45.35%, jiangsu hengrui medicine for gonow capsule YiYi occupies 22.95%, qilu pharmaceutical for gonow capsule account for 16.08%. The imported drugs, which are the equivalent of Japan's dapeng pharmaceuticals, account for 15.58%. The statistics show that jiangsu hengrui and qilu pharmaceuticals are showing sustained growth.
Capebera has benefited from the fake boom
Capectamine is a new generation of oral fluorouracil drugs developed by roche of Switzerland. It was approved by the FDA in April 1998 and is called Xeloda (hiroda). A first-line treatment for late-stage or metastatic gastric cancer that cannot be performed. In November 1999, he entered the Chinese clinic. On September 16, 2013, the F.D.A. approved a U.S. listing of the first copy of the drug, called "tava" by the FDA. His patents in China expired on December 17, 2013, leading to the imitation of the domestic card market.
In 2012 jiangsu hengrui pharmaceutical co. In December 2013, CFDA approved the registration of qilu's tablet, which was approved by CFDA in December 2014. So far, three domestic drugs have monopolized the carpe-bin market.
According to data from the HDM system, the amount of drug used by the public hospital in China's major urban public hospitals was 611 million yuan in 2016, up 13.64 percent year on year. With the listing of the locally made capotian bin, it will gradually break down the whole world of the original research drug hiroda.
Public hospital 2016 key cities for the domestic market, roche, account for 72.24% of the previous year fell by 10%, by three domestic capecitabine manufacturers, the brand of jiangsu hengrui YiBin occupies 11.09% share of qilu pharmaceutical brand ZhuoLun accounted for 9.19% of the share, zhengda sunny brand and records accounted for 7.47% of the share. The growth of three domestic brands is forecast for 2017.
Targeted therapies are emerging
The journal lancet oncology, so far, the poor prognosis of patients with advanced gastric cancer, is still missing some new scheme of effective treatment, treatment demand is far from being satisfied. At present, the target drug in the domestic gastric cancer drug treatment market is roche trastuzumab, jiangsu hengrui appatinib.
In October 2010, the FDA approved roche/genentech by bead single joint by fluorouracil and cisplatin resistance or capecitabine and cisplatin for the treatment of epidermal growth factor receptor - 2 (HER2 positive) gastric cancer. In 2012 CFDA approved trastuzumab for the first line of treatment for HER2 positive metastatic gastric cancer, with new options for late-stage gastric cancer treatment. But the HER2 sex ratio is very different, generally considered to be about 20 percent of the stomach cancer population, and on the other hand, the price of antibody drugs is expensive, and most people can't afford it. Therefore, the development of targeted therapies is still an important topic.
On October 17, 2014, jiangsu hengrui pharmaceutical co., LTD., a national 1.1 class of new drugs, was approved for the listing. This is another major breakthrough in the development of cancer treatment in our country.
According to HDM system, according to data from 2016 key cities for the domestic public hospital gastric cancer targeted drugs path for up to 69.15 million yuan, year-on-year growth of 18.37% a year, made a good beginning.
Path for Nepal is the world's first in advanced gastric cancer proved safe and effective targeted small molecule anti-angiogenesis drugs, and standardization of advanced gastric cancer after treatment failure, curative effect is the best medicine. At the same time, apatinib is the only oral preparation for gastric cancer targeted drugs that will greatly improve the adherence to treatment.
Path for is a new small molecular targeted drugs that inhibit tumor angiogenesis, which applies to cancer cells, can significantly extend the survival period of patients with advanced gastric cancer, greatly reduce the cost of patients at the same time. Apatinib is the national "eleventh five-year" and "twelfth five-year" major new drug initiative, and hengrui has four inventions, including two global patents.
As jiangsu hengrui path for the arrival of that market has formed a path for his stomach cancer treatment and capecitabine, gonow, oxaliplatin into, the more west yew and by bead on behalf of kang and monoclonal antibodies such as a new pattern of the combination, and make the gastric cancer and patients with advanced gastric cancer with hope.
With the global process of new drug research and development of accelerating, clinical medicine has become increasingly perfect, especially gastric cancer, colorectal cancer targeted drugs has become a hit to the world pharmaceutical market of heavy hammer. Antitumor drugs are a hot category that is diverse and ethnically diverse |
