Medical net May 27 is in the medical device industry, various regulation law and regulations are numerous, do not know what behavior is easy to be targeted by the drug administration department? I don't know what kind of punishment will be imposed if I make a mistake. In what circumstances can you be given a lighter or mitigated punishment? Which situations will be given the heavier punishment? How to get lighter and heavier? A new document from the shandong provincial drug administration can help you solve many puzzles at once.
On May 26, in shandong province food and drug administration announced "the shandong province food and drug administrative penalty discretion benchmark (medical devices) (draft)", on June 10, 2017 for the public to ask for some advice.
Shandong province food and drug administration of the "draft" punishment on the basis of content and variety are from medical devices regulations industry laws and regulations, such as can be seen as general standard of punishment, the same all over the country, also won't vary too much.
At the same time, the draft is in order to meet the national and provincial level since 2015 a series of new laws and regulations and organize revision, also represents a new trend of drug safety to exercise administrative penalty discretion.
There are 28 sports in the column "draft" of the communist party of China medical equipment production, management and use of violation behavior, a wide coverage, to say the medical instrument enterprises and medical organizations make daily questions one by one in the column. For industry and people, there is always one that fits you.
The following is a detailed description of the "price tag" fines for various offences:
(1) the production and operation of the second class of medical devices which have not obtained the registration certificate of medical instruments
Punishment measures include the confiscation of illegal income, medical devices that are illegally produced and operated and the tools, equipment and raw materials used in the illegal production and operation; Concurrently be imposed; If the circumstances are serious, it shall not accept the application for licensing of medical devices by the relevant persons or enterprises within five years; If the circumstances are serious, the original issuing department may revoke the license for the production of medical devices or medical devices.
Fine "price list" :
Engage in the production activities of type 2 and 3 medical devices without permission
Punishments include the confiscation of illegal income, medical devices illegally produced and the tools, equipment and raw materials used in the illegal production and operation; Concurrently be imposed; If the circumstances are serious, it shall not accept the application for licensing of medical devices by the relevant persons and enterprises within five years.
Fine price list:
To engage in the operation of a third type of medical device without permission
Punishment measures include the confiscation of illegal income, medical devices that are illegally produced and operated and the tools, equipment and raw materials used in the illegal production and operation; Concurrently be imposed; If the circumstances are serious, it shall not accept the application for licensing of medical devices by the relevant persons and enterprises within five years.
Fine price list:
4, providing false information or take other deception to obtain registration certificate of medical equipment, medical equipment production license, medical equipment business licenses, advertising and other documents of approval certificates
Penalties include: revoking permits; Concurrently be imposed; Within five years, the applicant shall not accept the application for the application of the medical instrument of the relevant persons and enterprises.
Fine price list:
Forgery, alteration, sale, lease and loan of related medical instruments
Punishments include: confiscation or revocation of the permit; Confiscation of illegal income; Fine; If the act constitutes a violation of public security administration, it shall be punished by the public security organ in accordance with the law.
Fine price list:
Failing to file in accordance with the regulations on the supervision and administration of medical devices
Punishment: a fine
Fine price list:
Provide false information for the record
Punishment: if the circumstances are serious, the direct responsibility personnel shall not engage in the production and operation of medical devices within five years
Eight, five major violations, including: production, management, use do not conform to the compulsory standards or does not conform to the product technical requirements for registration or registration of medical apparatus and instruments; Where the production enterprise fails to organize production according to the product technology registered or filed, or fails to establish the quality management system in accordance with the regulations and maintains the effective operation; Operation, use of unqualified documents, expired, invalidated, obsolete medical instruments, or use medical instruments that are not lawfully registered; If the drug administration is ordered, it shall still refuse to carry out the recall or stop the operation of medical devices; An enterprise that does not have the prescribed conditions to produce medical instruments or is not managed by the agent's production practices.
Punishments include the seizure of medical devices that have been illegally produced, operated or used; Concurrently be imposed; Order the cessation of production and suspension; Revoke the license of medical instrument registration certificate, medical device production license and medical device.
Fine price list:
9, four major violations, including: production conditions of enterprises change, no longer meet the demands of medical device quality management system, and not according to stipulations rectification, stop the production, the report; The production, operation instruction, and the label not conforming to the prescribed medical instruments; Failing to transport and store medical instruments according to the instructions and labels; Transfer of expired, expired, obsolete or unqualified to use medical instruments.
Penalties include: fines; Order the cessation of production and suspension; To revoke the license for production of medical devices and medical devices.
Fine price list:
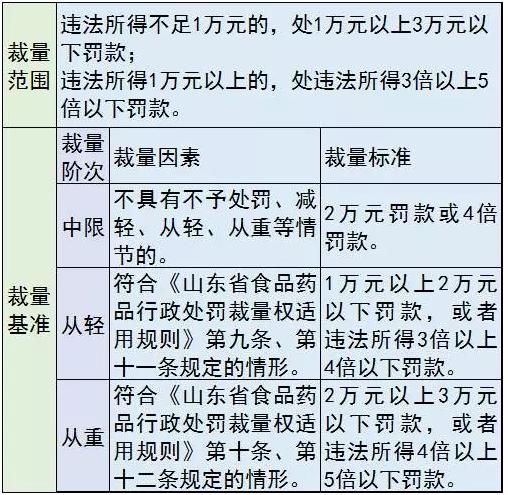
The illegal activities of the eight countries, including the failure of production enterprises to submit the quality management system according to the requirements of the report; Failing to establish and execute the inspection records system in accordance with the provisions of the relevant regulations; Category ii, third class medical device wholesalers and the third category of medical device retailers have not established and implemented the sales record system in accordance with the regulations; For regular inspection, testing, calibration, maintenance, maintenance of medical apparatus and instruments, using unit not in accordance with the requirements of the product data sheet for regular inspection, testing, calibration, maintenance, maintenance and record; The unit fails to properly preserve the raw data of the third category of medical devices; The use of records for the use of implanted and interventional medical devices by units that are not established and kept in accordance with the regulations; Unit using of using medical devices are found safe hidden trouble not immediately stop using, notifications, repair, or continue to use after repair still cannot achieve use safety standards of medical apparatus and instruments; Production and operation enterprises, use the unit is not in accordance with the relevant provisions in the medical device adverse event monitoring, did not report adverse events in accordance with the requirements, or the medical device adverse event monitoring technology institutions of adverse events, drug safety survey by the not cooperate.
Penalties include: warning; Fine; Order the cessation of production and suspension; To revoke the license for production of medical devices and medical devices.
Fine price list:
To carry out clinical trials of medical devices in violation of the regulations on the supervision and administration of medical devices
Penalties include: fines; Within five years, no clinical trial of relevant medical devices shall be carried out.
Fine price list:
The clinical trial institute of medical devices issues false reports
The punishments include: no clinical trial of relevant medical devices within 10 years; Confiscation of illegal income; A fine.
Fine price list:
Tampering with approved medical device advertising content
Punishment: to revoke the advertising approval document for the medical instrument; Within one year, the application for examination and approval is not accepted.
The advertisement for false medical devices was decided by the provincial bureau to suspend the sale of the medical instrument
Punishment: the confiscation of illegal medical devices; A fine.
Fine price list:
The applicant fails to conduct clinical trials in accordance with the regulations on the supervision and administration of medical devices and the measures for registration of medical devices
Punishment: a fine; Log out of clinical trial approval documents.
Fine price list:
The applicant fails to conduct clinical trials in accordance with the regulations on the supervision and administration of medical devices and the measures for the management of the registration of reagents in vitro
Punishment: a fine
Fine price list:
To forge, alter, buy and sell, lease, and lend medical devices to produce the record certificates
Punishment: a fine
Fine price list:
(6) the six ACTS of illegal activities, including the failure of the factory medical instrument to be inspected in accordance with the relevant provisions; If the factory medical instrument fails to be accompanied by a certificate of conformity with the relevant provisions, Failing to carry out the registration of changes in the production license for medical devices in accordance with the provisions; Failing to carry out the formalities for the production and filing of the entrusted production in accordance with the provisions; If the products of medical instruments have been shut down for more than one year without the same kind of products in production, they shall be returned to production without the verification of the local pharmaceutical supervision department; To conceal the relevant situation, provide false information or refuse to provide the actual information that reflects its activities in the supervision and inspection department.
Punishment: warning, fine
Fine price list:
The three major violations of the medical device operating enterprises, including: failing to handle the change of registration in accordance with the regulations governing the supervision and administration of medical devices; Where the enterprise sends personnel to sell medical instruments and fails to provide the authorization letter as required; The third type of medical device operating company does not submit annual report to the drug administration before the end of the year.
Punishment: warning; A fine.
Fine price list:
The four major illegal behaviors of the business enterprise, including: changes in the operating conditions of the enterprise, no longer conforming to the requirements of the GSP, and failing to comply with the regulations; To change the premises or the address of the warehouse, expand the scope of business or the establishment of a warehouse; Selling the business enterprise engaged in the wholesale business of medical devices to a non-qualified business enterprise or unit; Never having a qualified production or business enterprise to purchase medical instruments.
Punishment: a fine
Fine price list:
Forging, altering, buying and selling, leasing or lending medical devices for the record
Punishment: a fine
Fine price list:

22, medical equipment unit using eight major violations, including: equipped with corresponding to the scale of its not according to stipulations or quality management of medical device quality management staff, or not according to stipulations established quality management of the use of the whole process of quality management system; Failing to purchase medical instruments uniformly by designated departments or personnel; Purchase, use the first type of medical instrument that has not been put on record, or the business enterprise that has never been put on record to purchase the second type of medical instrument; Storage place for medical equipment and medical equipment, facilities and conditions, quantity does not adapt, or not in accordance with the requirements for storage conditions, effective period to regularly check and record storage of medical apparatus and instruments; Failing to establish and implement the system of quality inspection before the use of medical instruments; The records of maintenance and maintenance of medical devices are not taken or kept in accordance with regulations; Failing to conduct the training and evaluation of the relevant technical personnel engaged in the maintenance and maintenance of medical devices in accordance with the provisions of the regulations, and establish the training archives; Not to check the quality management of its medical equipment according to the regulations, and make self-examination report.
Punishment: warning; Those who refuse to make corrections shall be fined.
Fine price list:
23, medical device manufacturing enterprise in violation of regulations, failure to provide maintenance services, or not according to requirements of materials and information necessary to provide maintenance service.
Punishment: warning; If the circumstances are serious or refuse to be corrected, a fine shall be imposed.
Fine price list:
24, medical equipment, production and operation enterprises and units used maintenance services, such as don't cooperate with the drug safety supervision and inspection, or reject, hide, not truthfully provide relevant information and data.
Punishment: warning; You can also pay a fine.
Fine price list:
The four major violations of the law include: failing to issue product recall information to the society in a timely manner; Failing to notify the business enterprise, the unit of use or inform the user within the prescribed time limit; Failing to adopt corrective measures or re-recall medical instruments according to the requirements of the drug administration; No detailed records of the recall or report to the drug administration.
Punishment: warning; A fine.
Fine price list:
The four major illegal activities, including the failure to establish a recall management system for medical devices in accordance with the regulations; Refusing to cooperate with the investigation of the drug administration; Failing to submit the report of the recall, the investigation assessment report and the recall plan, the implementation of the recall plan and the report of the summary assessment in the absence of the provisions; The plan for the change of the recall shall not be reported to the drug administration for the record.
Punishment: warning; Where the overdue correction is not made, a fine shall be imposed.
Fine price list:
Failing to suspend the sale or use the product immediately; Failing to inform the production enterprise or supplier in time; Do not report to the local provincial pharmaceutical or health administration department.
Punishment: order to stop selling and use medical instruments that are defective; Fine; If serious consequences are caused, the license for operation of medical devices shall be revoked.
Fine price list:
Refusing to cooperate with the investigation of defects in medical devices; Refusing to assist production enterprises to recall medical instruments.
Punishment: warning; If he refuses to correct, he shall be fined.
Fine price list:
|
