Medical network - on December 28, ningxia hui autonomous region of the medical equipment product registration fee charging standard "officially released. Surprisingly, the food and drug administration for the second category of medical equipment product registration charge RMB 31300 for the first time, the change and continuity registration charges 13000 yuan only, too! It has brought in a rate of 10 provinces, is an unprecedented low.
Ningxia in the second category of medical device product registration specific charges:
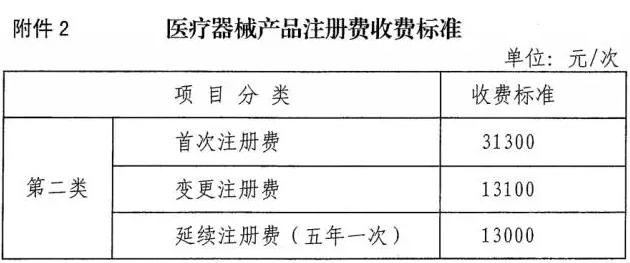
Medical equipment product registration fee according to the measures for the administration of the medical device registration, the in vitro diagnostic reagents registration measures for the administration of registration unit instead.
The medical device registration measures for the administration of the registration measures for the management of in vitro diagnostic reagent, belong to the record of the login to apply for a change of circumstances, is not for change registration fee.
Small micro enterprise charge preferential policies according to the state administration of drug safety announcement no. 53 (2015) regulations.
The above provisions since January 1, 2017 implementation, trial period of 2 years.
Big contrast: ningxia VS other provinces charge standard
Previously, the domestic existing in fujian, jiangxi, Shanghai, hainan, shandong, zhejiang, anhui, shaanxi, Inner Mongolia, Beijing, jilin, hebei, jiangsu successively issued in the second category of medical device product registration fees, ningxia is the 14th for province medical equipment product registration fee charging standard.
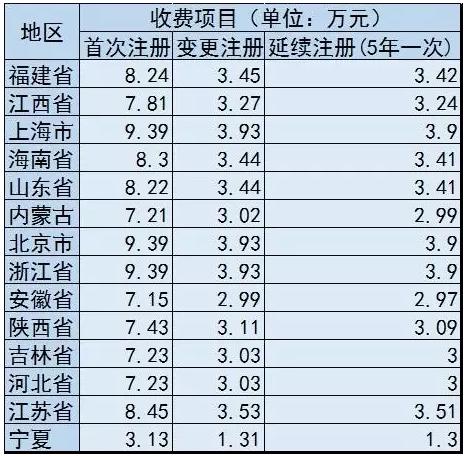
|
